How Many Electrons Can the Second Orbital Hold
In chemistry and atomic physics, an electron shell may exist thought of as an orbit followed past electrons effectually an atom'due south nucleus. The closest shell to the nucleus is called the "one shell" (also called the "K beat"), followed by the "2 shell" (or "L trounce"), then the "three beat out" (or "Yard beat out"), and so on farther and farther from the nucleus. The shells correspond to the chief quantum numbers (north = 1, 2, 3, 4 ...) or are labeled alphabetically with the letters used in X-ray note (1000, L, M, …).
Each shell can contain only a fixed number of electrons: The first vanquish can hold upwards to two electrons, the second shell tin can hold up to viii (2 + 6) electrons, the third shell tin hold upwardly to 18 (2 + 6 + x) and so on. The general formula is that the due northth vanquish can in principle hold upwardly to 2(due north 2) electrons.[1] For an explanation of why electrons exist in these shells see electron configuration.[2]
Each shell consists of 1 or more subshells, and each subshell consists of one or more atomic orbitals.
History
The 1913 Bohr model of the atom attempted an organization of electrons in their sequential orbits, even so, at that time Bohr continued to increase the inner orbit of the cantlet to 8 electrons as the atoms got larger. Bohr built his 1913 model of electrons in elements thus:[three]
"From the above we are led to the following possible scheme for the arrangement of the electrons in light atoms:
| Element | Electrons per trounce |
|---|---|
| 4 | 2, 2 |
| 6 | two, 4 |
| 7 | four, 3 |
| eight | 4, ii, 2 |
| 9 | 4, four, 1 |
| 10 | 8, 2 |
| xi | 8, 2, 1 |
| 16 | 8, 4, 2, two |
| 18 | eight, 8, two |
Periodic tabular array of Bohr in 1913 showing electron configurations in his second paper where he went to the 24th element.[4] [5]
The beat terminology comes from Arnold Sommerfeld'southward modification of the Bohr model. During this period Bohr was working with Walther Kossel, whose papers in 1914 and in 1916 chosen the orbits "shells."[6] [vii] Sommerfeld retained Bohr's planetary model, but added mildly elliptical orbits (characterized past additional quantum numbers ℓ and m) to explain the fine spectroscopic structure of some elements.[8] The multiple electrons with the aforementioned master quantum number (n) had close orbits that formed a "shell" of positive thickness instead of the circular orbit of Bohr'southward model which orbits called "rings" were described by a plane.[9]
The existence of electron shells was outset observed experimentally in Charles Barkla's and Henry Moseley's 10-ray assimilation studies. Moseley's piece of work did non directly concern the study of electron shells, because he was trying to bear witness that the periodic table was not bundled by weight, only past the charge of the protons in the nucleus.[x] However, because in a neutral atom, the number of electrons equals the number of protons, this work was extremely of import to Niels Bohr who mentioned Moseley'due south work several times in his interview of 1962.[11] Moseley was part of Rutherford'southward grouping, as was Niels Bohr. Moseley measured the frequencies of X-rays emitted by every element betwixt calcium and zinc, and found that the frequencies became greater as the elements got heavier, leading to the theory that electrons were emitting Ten-rays when they were shifted to lower shells.[12] This led to the conclusion that the electrons were in Kossel's shells with a definite limit per trounce, labeling the shells with the letters K, L, Thousand, N, O, P, and Q.[thirteen] [14] The origin of this terminology was alphabetic. Barkla, who worked independently from Moseley as an X-ray spectrometry experimentalist, first noticed two singled-out types of scattering from shooting Ten-rays at elements in 1909 and named them "A" and "B". Barkla described these two types of X-ray diffraction: the first was unconnected with the type of material used in the experiment, and could be polarized. The other second diffraction beam he called "fluorescent" because information technology depended on the irradiated fabric.[xv] It was not known what these lines meant at the time, simply in 1911 Barkla decided in that location might be handful lines previous to "A", and so he began at "K".[16] Nonetheless, later experiments indicated that the K absorption lines are produced by the innermost electrons. These messages were later on found to correspond to the northward values i, 2, 3, etc. that were used in the Bohr model. They are used in the spectroscopic Siegbahn notation.
The piece of work of assigning electrons to shells was continued from 1913 to 1925 past many chemists and a few physicists. Niels Bohr was one of the few physicists who followed the chemist'south work[17] of defining the periodic table, while Arnold Sommerfeld worked more than on trying to make a relativistic working model of the atom that would explain the fine construction of the spectra from a classical orbital physics standpoint through the Atombau approach.[18] Einstein and Rutherford, who did not follow chemistry, were unaware of the chemists who were developing electron crush theories of the periodic tabular array from a chemistry point of view, such equally Irving Langmuir, Charles Bury, J.J. Thomson, and Gilbert Lewis, who all introduced corrections to Bohr's model such equally a maximum of ii electrons in the first vanquish, 8 in the side by side and so on, and were responsible for explaining valency in the outer electron shells, and the edifice up of atoms by calculation electrons to the outer shells.[xix] [20] And then when Bohr outlined his electron shell diminutive theory in 1922, there was no mathematical formula for the theory. So Rutherford said he was hard put "to course an idea of how y'all arrive at your conclusions".[21] [22] Einstein said of Bohr'south 1922 paper that his "electron-shells of the atoms together with their significance for chemical science appeared to me like a miracle – and appears to me equally a miracle fifty-fifty today".[23] Arnold Sommerfeld, who had followed the Atombau structure of electrons instead of Bohr who was familiar with the chemists' views of electron structure, spoke of Bohr's 1921 lecture and 1922 commodity on the shell model every bit "the greatest advance in atomic structure since 1913".[24] [25] [26] However, the electron shell development of Niels Bohr was basically the same theory as that of the chemist Charles Rugeley Bury in his 1921 paper.[27] [28] [29]
Every bit work continued on the electron crush structure of the Sommerfeld-Bohr Model, Sommerfeld had introduced three "quantum numbers north, one thousand, and one thousand, that described the size of the orbit, the shape of the orbit, and the direction in which the orbit was pointing."[30] Because we use k for the Boltzmann constant, the azimuthal breakthrough number was changed to ℓ. When the mod quantum mechanics theory was put forward based on Heisenberg'south matrix mechanics and Schrödinger's moving ridge equation, these quantum numbers were kept in the current quantum theory just were changed to n being the principal quantum number, and m beingness the magnetic quantum number.
However, the final grade of the electron shell model still in use today for the number of electrons in shells was discovered in 1923 past Edmund Stoner, who introduced the principle that the nth shell was described by 2(n 2). Seeing this in 1925, Wolfgang Pauli added a quaternary quantum number, "spin", during the old quantum theory menstruation of the Sommerfeld-Bohr solar organization atom to complete the mod electron shell theory.[31]
Subshells
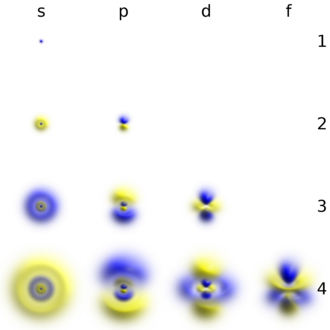
Each shell is composed of ane or more subshells, which are themselves equanimous of atomic orbitals. For example, the first (K) beat out has ane subshell, called 1s; the 2nd (L) shell has ii subshells, called 2s and 2p; the third shell has 3s, 3p, and 3d; the fourth shell has 4s, 4p, 4d and 4f; the fifth shell has 5s, 5p, 5d, and 5f and tin theoretically hold more than in the 5g subshell that is not occupied in the footing-state electron configuration of whatsoever known element.[2] The diverse possible subshells are shown in the following table:
| Subshell label | ℓ | Max electrons | Shells containing it | Historical name |
|---|---|---|---|---|
| southward | 0 | ii | Every shell | due southharp |
| p | ane | half dozen | 2nd vanquish and higher | principal |
| d | 2 | x | 3rd crush and higher | diffuse |
| f | 3 | 14 | 4th beat out and college | fundamental |
| g | iv | 18 | 5th trounce and higher (theoretically) | (next in alphabet after f) [32] |
- The first column is the "subshell label", a lowercase-letter label for the blazon of subshell. For instance, the "4s subshell" is a subshell of the 4th (North) beat, with the blazon (s) described in the beginning row.
- The second column is the azimuthal breakthrough number (ℓ) of the subshell. The precise definition involves quantum mechanics, but information technology is a number that characterizes the subshell.
- The 3rd column is the maximum number of electrons that can be put into a subshell of that type. For example, the top row says that each due south-type subshell (1s, 2s, etc.) tin can have at most two electrons in it. In each example the figure is 4 greater than the 1 above information technology.
- The fourth column says which shells have a subshell of that type. For example, looking at the tiptop two rows, every shell has an s subshell, while only the 2d beat out and higher have a p subshell (i.e., there is no "1p" subshell).
- The terminal cavalcade gives the historical origin of the labels south, p, d, and f. They come from early studies of atomic spectral lines. The other labels, namely g, h and i, are an alphabetic continuation following the concluding historically originated label of f.
Number of electrons in each shell
Each subshell is constrained to concur ivℓ + 2 electrons at well-nigh, namely:
- Each s subshell holds at most 2 electrons
- Each p subshell holds at nigh half dozen electrons
- Each d subshell holds at almost 10 electrons
- Each f subshell holds at most 14 electrons
- Each g subshell holds at most eighteen electrons
Therefore, the K beat, which contains but an south subshell, tin can hold up to 2 electrons; the L shell, which contains an s and a p, can concord upwardly to 2 + six = 8 electrons, and then forth; in general, the northth shell can hold upwards to iin 2 electrons.[1]
| Shell name | Subshell proper name | Subshell max electrons | Beat max electrons |
|---|---|---|---|
| K | 1s | two | 2 |
| 50 | 2s | two | 2 + 6 = 8 |
| 2p | half dozen | ||
| Thousand | 3s | ii | ii + 6 + 10 = 18 |
| 3p | vi | ||
| 3d | ten | ||
| Northward | 4s | ii | 2 + 6 + x + 14 = 32 |
| 4p | 6 | ||
| 4d | 10 | ||
| 4f | fourteen | ||
| O | 5s | 2 | 2 + 6 + 10 + 14 + 18 = 50 |
| 5p | vi | ||
| 5d | 10 | ||
| 5f | 14 | ||
| 5g | xviii |
Although that formula gives the maximum in principle, in fact that maximum is only achieved (in known elements) for the kickoff four shells (K, L, M, N). No known chemical element has more than 32 electrons in any one shell.[33] [34] This is because the subshells are filled according to the Aufbau principle. The get-go elements to have more than 32 electrons in one beat out would belong to the g-cake of menses eight of the periodic tabular array. These elements would accept some electrons in their 5g subshell and thus have more than 32 electrons in the O shell (fifth principal beat out).
Subshell energies and filling club

For multielectron atoms n is a poor indicator of electron'south energy. Energy spectra of some shells interleave.
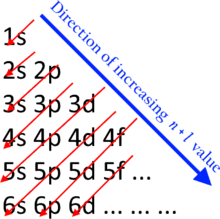
The states crossed by same cherry-red arrow accept aforementioned value. The direction of the scarlet arrow indicates the lodge of land filling.
Although it is sometimes stated that all the electrons in a shell have the aforementioned energy, this is an approximation. Yet, the electrons in one subshell do accept exactly the same level of energy, with afterward subshells having more than free energy per electron than earlier ones. This issue is keen enough that the energy ranges associated with shells can overlap.
The filling of the shells and subshells with electrons gain from subshells of lower energy to subshells of college free energy. This follows the n + ℓ rule which is as well usually known as the Madelung dominion. Subshells with a lower northward + ℓ value are filled before those with higher due north + ℓ values. In the case of equal n + ℓ values, the subshell with a lower n value is filled first.
List of elements with electrons per shell
The list below gives the elements arranged past increasing diminutive number and shows the number of electrons per crush. At a glance, the subsets of the list show obvious patterns. In particular, every set of five elements (in electric blue) earlier each element of group 0 (group 18, in yellow) heavier than helium accept successive numbers of electrons in the outermost shell, namely three to 7.
Sorting the table by chemical grouping shows additional patterns, especially with respect to the last two outermost shells. (Elements 57 to 71 belong to the lanthanides, while 89 to 103 are the actinides.)
The list below is primarily consistent with the Aufbau principle. Notwithstanding, in that location are a number of exceptions to the dominion; for example palladium (atomic number 46) has no electrons in the fifth shell, dissimilar other atoms with lower diminutive number. Some entries in the table are uncertain, when experimental data is unavailable. (For instance, the elements past 108 have such short half-lives that their electron configurations take not yet been measured.)
| Z | Element | No. of electrons/vanquish | Grouping |
|---|---|---|---|
| one | Hydrogen | 1 | 1 |
| 2 | Helium | 2 | xviii |
| iii | Lithium | 2, ane | 1 |
| 4 | Beryllium | 2, two | 2 |
| v | Boron | ii, three | 13 |
| vi | Carbon | 2, iv | fourteen |
| 7 | Nitrogen | 2, five | 15 |
| viii | Oxygen | 2, 6 | xvi |
| 9 | Fluorine | ii, 7 | 17 |
| 10 | Neon | ii, 8 | eighteen |
| 11 | Sodium | two, 8, 1 | 1 |
| 12 | Magnesium | 2, 8, ii | 2 |
| xiii | Aluminium | two, eight, three | thirteen |
| 14 | Silicon | 2, 8, 4 | 14 |
| 15 | Phosphorus | ii, eight, 5 | 15 |
| 16 | Sulfur | ii, 8, half dozen | 16 |
| 17 | Chlorine | 2, 8, seven | 17 |
| eighteen | Argon | ii, eight, 8 | xviii |
| 19 | Potassium | 2, 8, 8, ane | i |
| 20 | Calcium | 2, viii, viii, 2 | 2 |
| 21 | Scandium | 2, 8, 9, 2 | 3 |
| 22 | Titanium | 2, 8, 10, 2 | 4 |
| 23 | Vanadium | 2, 8, 11, two | 5 |
| 24 | Chromium | 2, eight, 13, 1 | vi |
| 25 | Manganese | ii, 8, 13, ii | seven |
| 26 | Iron | 2, eight, 14, 2 | 8 |
| 27 | Cobalt | ii, 8, 15, 2 | nine |
| 28 | Nickel | two, 8, xvi, ii | ten |
| 29 | Copper | 2, 8, 18, ane | 11 |
| 30 | Zinc | two, 8, xviii, 2 | 12 |
| 31 | Gallium | 2, 8, eighteen, three | 13 |
| 32 | Germanium | 2, eight, xviii, 4 | 14 |
| 33 | Arsenic | ii, 8, 18, 5 | 15 |
| 34 | Selenium | 2, 8, 18, six | sixteen |
| 35 | Bromine | 2, 8, 18, 7 | 17 |
| 36 | Krypton | 2, viii, 18, eight | 18 |
| 37 | Rubidium | 2, 8, eighteen, viii, i | 1 |
| 38 | Strontium | two, 8, xviii, eight, 2 | 2 |
| 39 | Yttrium | 2, 8, xviii, ix, two | 3 |
| 40 | Zirconium | 2, 8, 18, 10, 2 | 4 |
| 41 | Niobium | two, 8, 18, 12, 1 | 5 |
| 42 | Molybdenum | 2, 8, eighteen, thirteen, 1 | vi |
| 43 | Technetium | two, eight, 18, 13, 2 | 7 |
| 44 | Ruthenium | 2, 8, 18, 15, 1 | 8 |
| 45 | Rhodium | two, 8, eighteen, 16, one | ix |
| 46 | Palladium | 2, 8, eighteen, 18 | 10 |
| 47 | Argent | ii, 8, eighteen, 18, 1 | eleven |
| 48 | Cadmium | 2, viii, 18, 18, two | 12 |
| 49 | Indium | two, viii, 18, 18, 3 | thirteen |
| l | Tin | 2, eight, 18, eighteen, 4 | 14 |
| 51 | Antimony | two, 8, 18, 18, 5 | 15 |
| 52 | Tellurium | 2, 8, 18, 18, 6 | sixteen |
| 53 | Iodine | 2, 8, 18, 18, 7 | 17 |
| 54 | Xenon | 2, 8, eighteen, 18, 8 | 18 |
| 55 | Caesium | 2, 8, xviii, xviii, 8, 1 | 1 |
| 56 | Barium | 2, eight, 18, 18, 8, ii | ii |
| 57 | Lanthanum | 2, viii, 18, 18, 9, 2 | |
| 58 | Cerium | 2, 8, 18, 19, 9, 2 | |
| 59 | Praseodymium | 2, 8, 18, 21, viii, 2 | |
| lx | Neodymium | 2, eight, 18, 22, 8, two | |
| 61 | Promethium | 2, eight, 18, 23, 8, 2 | |
| 62 | Samarium | 2, 8, 18, 24, 8, 2 | |
| 63 | Europium | two, eight, xviii, 25, 8, ii | |
| 64 | Gadolinium | 2, 8, 18, 25, 9, 2 | |
| 65 | Terbium | 2, 8, 18, 27, 8, 2 | |
| 66 | Dysprosium | 2, viii, xviii, 28, 8, 2 | |
| 67 | Holmium | 2, 8, 18, 29, 8, 2 | |
| 68 | Erbium | 2, viii, 18, xxx, 8, ii | |
| 69 | Thulium | 2, eight, 18, 31, 8, 2 | |
| 70 | Ytterbium | 2, 8, 18, 32, 8, 2 | |
| 71 | Lutetium | 2, 8, 18, 32, 9, 2 | 3 |
| 72 | Hafnium | two, eight, xviii, 32, ten, 2 | 4 |
| 73 | Tantalum | two, 8, eighteen, 32, 11, two | 5 |
| 74 | Tungsten | 2, 8, 18, 32, 12, 2 | 6 |
| 75 | Rhenium | 2, 8, xviii, 32, 13, 2 | 7 |
| 76 | Osmium | two, 8, eighteen, 32, xiv, two | 8 |
| 77 | Iridium | 2, 8, 18, 32, 15, 2 | 9 |
| 78 | Platinum | 2, eight, xviii, 32, 17, ane | ten |
| 79 | Gilt | 2, 8, eighteen, 32, eighteen, 1 | eleven |
| 80 | Mercury | 2, viii, 18, 32, 18, 2 | 12 |
| 81 | Thallium | ii, eight, 18, 32, 18, iii | 13 |
| 82 | Lead | 2, 8, eighteen, 32, 18, 4 | 14 |
| 83 | Bismuth | 2, 8, eighteen, 32, 18, five | 15 |
| 84 | Polonium | 2, 8, 18, 32, 18, six | 16 |
| 85 | Astatine | 2, 8, xviii, 32, eighteen, 7 | 17 |
| 86 | Radon | 2, eight, 18, 32, 18, 8 | xviii |
| 87 | Francium | 2, viii, 18, 32, xviii, viii, one | ane |
| 88 | Radium | ii, 8, 18, 32, 18, 8, 2 | two |
| 89 | Actinium | 2, 8, xviii, 32, eighteen, 9, 2 | |
| ninety | Thorium | 2, eight, eighteen, 32, eighteen, 10, 2 | |
| 91 | Protactinium | 2, 8, 18, 32, 20, 9, 2 | |
| 92 | Uranium | ii, 8, eighteen, 32, 21, 9, 2 | |
| 93 | Neptunium | 2, viii, xviii, 32, 22, 9, 2 | |
| 94 | Plutonium | two, 8, 18, 32, 24, 8, 2 | |
| 95 | Americium | 2, eight, 18, 32, 25, 8, 2 | |
| 96 | Curium | two, viii, eighteen, 32, 25, 9, ii | |
| 97 | Berkelium | 2, 8, 18, 32, 27, 8, 2 | |
| 98 | Californium | 2, 8, 18, 32, 28, eight, 2 | |
| 99 | Einsteinium | ii, 8, 18, 32, 29, 8, 2 | |
| 100 | Fermium | 2, eight, 18, 32, 30, 8, two | |
| 101 | Mendelevium | 2, eight, 18, 32, 31, viii, ii | |
| 102 | Nobelium | 2, eight, 18, 32, 32, 8, 2 | |
| 103 | Lawrencium | 2, 8, 18, 32, 32, eight, 3 | 3 |
| 104 | Rutherfordium | 2, 8, eighteen, 32, 32, ten, ii | iv |
| 105 | Dubnium | 2, 8, 18, 32, 32, 11, two | v |
| 106 | Seaborgium | 2, viii, 18, 32, 32, 12, 2 | 6 |
| 107 | Bohrium | 2, 8, eighteen, 32, 32, thirteen, 2 | 7 |
| 108 | Hassium | 2, viii, 18, 32, 32, 14, two | 8 |
| 109 | Meitnerium | 2, 8, 18, 32, 32, 15, two (?) | ix |
| 110 | Darmstadtium | ii, viii, eighteen, 32, 32, 16, ii (?) | 10 |
| 111 | Roentgenium | 2, eight, eighteen, 32, 32, 17, two (?) | 11 |
| 112 | Copernicium | two, eight, 18, 32, 32, xviii, two (?) | 12 |
| 113 | Nihonium | 2, eight, 18, 32, 32, 18, three (?) | xiii |
| 114 | Flerovium | two, viii, 18, 32, 32, 18, 4 (?) | 14 |
| 115 | Moscovium | ii, eight, xviii, 32, 32, 18, v (?) | 15 |
| 116 | Livermorium | 2, 8, 18, 32, 32, 18, 6 (?) | 16 |
| 117 | Tennessine | 2, 8, xviii, 32, 32, 18, 7 (?) | 17 |
| 118 | Oganesson | 2, viii, xviii, 32, 32, eighteen, 8 (?) | 18 |
See also
- Periodic table (electron configurations)
- Electron counting
- eighteen-electron rule
- Core charge
References
- ^ a b Re: Why do electron shells accept set limits ? madsci.org, 17 March 1999, Dan Berger, Faculty Chemical science/Science, Bluffton College
- ^ a b Electron Subshells. Corrosion Source.
- ^ Encounter Wikipedia periodic table.
- ^ Niels Bohr, "On the Constitution of Atoms and Molecules, Office Ii Systems containing just a Single Nucleus Philosophical Mag 26:857--875 (1913)
- ^ Kragh, Helge. "Niels Bohr's Second Diminutive Theory." Historical Studies in the Physical Sciences, vol. ten, University of California Press, 1979, pp. 123–86, https://doi.org/10.2307/27757389.
- ^ W. Kossel, "Über Molekülbildung als Folge des Atombaues," Ann. Phys., 1916, 49, 229-362 (237).
- ^ Translated in Helge Kragh, Aarhus, LARS VEGARD, Atomic Structure, AND THE PERIODIC SYSTEM, Balderdash. Hist. Chem., Volume 37, Number 1 (2012), p.43.
- ^ Donald Sadoway, Introduction to Solid Country Chemistry, Lecture 5 Archived 29 June 2011 at the Wayback Machine
- ^ Bohr, Niels (1913). On the Constitution of Atoms and Molecules, Function I. _Philosophical Magazine_ 26:1--25.
- ^ Uhler, Horace Scudder. "On Moseley's Law for Ten-Ray Spectra." Proceedings of the National Academy of Sciences of the Usa of America, vol. 3, no. ii, National Academy of Sciences, 1917, pp. 88–90, http://www.jstor.org/stable/83748.
- ^ Niels Bohr interview 1962 Session Three https://world wide web.aip.org/history-programs/niels-bohr-library/oral-histories/4517-3
- ^ Kumar, Manjit. Breakthrough: Einstein, Bohr, and the great debate about the nature of reality / Manjit Kumar.—1st American ed., 2008. Chap.4.
- ^ Kragh, Helge. "Niels Bohr's Second Atomic Theory." Historical Studies in the Concrete Sciences, vol. 10, University of California Printing, 1979, pp. 123–86, https://doi.org/10.2307/27757389.
- ^ Barkla, Charles G. (1911). "XXXIX.The spectra of the fluorescent Röntgen radiations". Philosophical Mag. Series half dozen. 22 (129): 396–412. doi:ten.1080/14786440908637137.
Previously denoted past messages B and A (...). The messages Chiliad and L are, still, preferable, as it is highly probable that series of radiations both more absorbable and more than penetrating exist.
- ^ Michael Eckert, Disputed discovery: the beginnings of 10-ray diffraction in crystals in 1912 and its repercussions, Jan 2011, Acta crystallographica. Section A, Foundations of crystallography 68(one):thirty-39 This Laue centennial article has also been published in Zeitschrift für Kristallographie [Eckert (2012). Z. Kristallogr. 227 , 27–35].
- ^ Charles G. Barkla M.A. D.Sc. (1911) XXXIX. The spectra of the fluorescent Röntgen radiations, The London, Edinburgh, and Dublin Philosophical Mag and Journal of Science, 22:129, 396-412, DOI: x.1080/14786440908637137
- ^ T.Hirosigeand S.Nisio,"Formation of Bohr's Theory of Atomic Constitution",Jap. Stud.Hist.Set.,No. three(1964),6-28.
- ^ Kragh, Helge. "Niels Bohr'due south Second Diminutive Theory." Historical Studies in the Concrete Sciences, vol. x, Academy of California Press, 1979, pp. 123–86, https://doi.org/10.2307/27757389.
- ^ Run into Periodic Table for full history.
- ^ Kragh, Helge. "Niels Bohr's 2d Diminutive Theory." Historical Studies in the Physical Sciences, vol. x, University of California Press, 1979, pp. 123–86, https://doi.org/10.2307/27757389.
- ^ Niels Bohr Nerveless Works, Vol. 4, p. 740. Postcard from Arnold Sommerfeld to Bohr, 7 March 1921.
- ^ Pais, Abraham (1991), Niels Bohr'south Times, in Physics, Philosophy, and Polity (Oxford: Clarendon Press), quoted p. 205.
- ^ Schilpp, Paul A. (ed.) (1969), Albert Einstein: Philosopher-Scientist (New York: MJF Books). Drove first published in 1949 as Vol. VII in the series The Library of Living Philosophers by Open up Court, La Salle, IL, Einstein, Albert 'Autobiographical Notes', pp.45-47.
- ^ Kragh, Helge. "Niels Bohr's Second Atomic Theory." Historical Studies in the Physical Sciences, vol. x, University of California Printing, 1979, pp. 123–86, https://doi.org/10.2307/27757389.
- ^ Kumar, Manjit. Quantum: Einstein, Bohr, and the nifty contend near the nature of reality / Manjit Kumar.—1st American ed., 2008. Chap.7.
- ^ Niels Bohr Collected Works, Vol. 4, p. 740. Postcard from Arnold Sommerfeld to Bohr, seven March 1921.
- ^ Bury, Charles R. (July 1921). "Langmuir's Theory of the Organization of Electrons in Atoms and Molecules". Journal of the American Chemical Order. 43 (7): 1602–1609. doi:10.1021/ja01440a023. ISSN 0002-7863.
- ^ Kragh, Helge. "Niels Bohr's 2nd Atomic Theory." Historical Studies in the Physical Sciences, vol. 10, University of California Press, 1979, pp. 123–86, https://doi.org/x.2307/27757389.
- ^ The Genesis of the Bohr Atom, John L. Heilbron and Thomas Due south. Kuhn, Historical Studies in the Concrete Sciences, Vol. 1 (1969), pp. vi, 211-290 (81 pages), Academy of California Printing,p. 285-286.
- ^ Kumar, Manjit. Quantum: Einstein, Bohr, and the great fence about the nature of reality / Manjit Kumar.—1st American ed., 2008. Chap.5.
- ^ Kragh, Helge. "Niels Bohr'due south Second Atomic Theory." Historical Studies in the Physical Sciences, vol. 10, University of California Printing, 1979, pp. 123–86, https://doi.org/10.2307/27757389.
- ^ Jue, T. (2009). "Breakthrough Mechanic Basic to Biophysical Methods". Fundamental Concepts in Biophysics. Berlin: Springer. p. 33. ISBN978-1-58829-973-4.
- ^ Orbitals. Chem4Kids. Retrieved on 1 December 2011.
- ^ Electron & Shell Configuration Archived 28 December 2022 at the Wayback Machine. Chemistry.patent-invent.com. Retrieved on ane December 2011.
Source: https://en.wikipedia.org/wiki/Electron_shell

Post a Comment for "How Many Electrons Can the Second Orbital Hold"